Smallpox
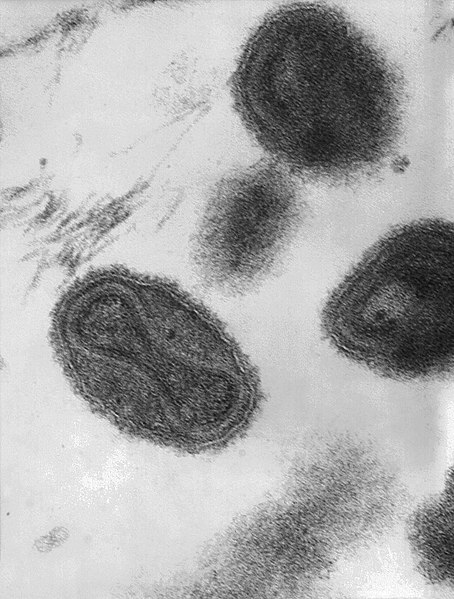
Smallpox was an infectious disease caused by either of two virus variants, Variola major and Variola minor.
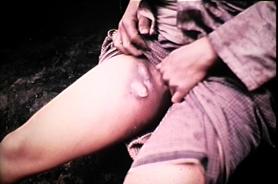
Smallpox was localized in small blood vessels of the skin and in the mouth and throat. In the skin it resulted in a characteristic maculopapular rash and, later, raised fluid-filled blisters. V. major produced a more serious disease and had an overall mortality rate of 30–35%. V. minor caused a milder form of disease, which killed about 1% of its victims. Long-term complications of V. major infection included characteristic scars, commonly on the face, which occur in 65–85% of survivors. Blindness resulting from corneal ulceration and scarring, and limb deformities due to arthritis and osteomyelitis were less common complications, seen in about 2–5% of cases.Smallpox is believed to have emerged in human populations about 10,000 BC. The earliest physical evidence of it is probably the pustular rash on the mummified body of Pharaoh Ramses V of Egypt. The disease killed an estimated 400,000 Europeans annually during the closing years of the 18th century (including five reigning monarchs), and was responsible for a third of all blindness. Of all those infected, 20–60%—and over 80% of infected children—died from the disease. Smallpox was responsible for an estimated 300–500 million deaths during the 20th century. As recently as 1967, the World Health Organization (WHO) estimated that 15 million people contracted the disease and that two million died in that year.
Signs and symptoms:
The incubation period between contraction and the first obvious symptoms of the disease is around 12 days. Once inhaled, variola major virus invades the oropharyngeal (mouth and throat) or the respiratory mucosa, migrates to regional lymph nodes, and begins to multiply. In the initial growth phase the virus seems to move from cell to cell, but around the 12th day, lysis of many infected cells occurs and the virus is found in the bloodstream in large numbers and a second wave of multiplication occurs in the spleen, bone marrow, and lymph nodes. The initial or prodromal symptoms are similar to other viral diseases such as influenza and the common cold: fever of at least 38.5 °C (101 °F), muscle pain, malaise, headache and prostration. As the digestive tract is commonly involved, nausea and vomiting and backache often occur. The prodrome, or preeruptive stage, usually lasts 2–4 days. By days 12–15 the first visible lesions—small reddish spots called enanthem—appear on mucous membranes of the mouth, tongue, palate, and throat, and temperature falls to near normal. These lesions rapidly enlarge and rupture, releasing large amounts of virus into the saliva
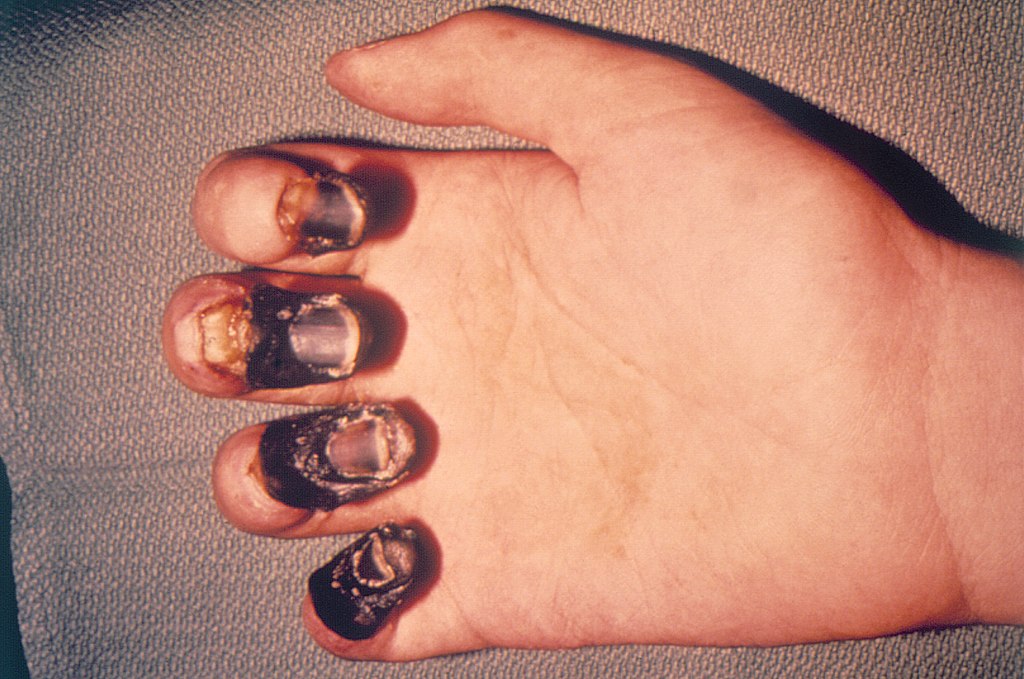
Hemorrhagic:
Hemorrhagic smallpox is a severe form that is accompanied by extensive bleeding into the skin, mucous membranes, and gastrointestinal tract. This form develops in approximately 2% of infections and occurred mostly in adults. In hemorrhagic smallpox the skin does not blister, but remains smooth. Instead, bleeding occurs under the skin, making it look charred and black, hence this form of the disease is also known as black pox.
Transimission:
Transmission occurs through inhalation of airborne variola virus, usually droplets expressed from the oral, nasal, or pharyngeal mucosa of an infected person. It is transmitted from one person to another primarily through prolonged face-to-face contact with an infected person, usually within a distance of 6 feet (1.8 m), but can also be spread through direct contact with infected bodily fluids or contaminated objects (fomites) such as bedding or clothing. Rarely, smallpox has been spread by virus carried in the air in enclosed settings such as buildings, buses, and trains. Smallpox is highly contagious, but generally spreads more slowly and less widely than some other viral diseases, perhaps because transmission requires close contact and occurs after the onset of the rash.
Treatment:
Smallpox vaccination within three days of exposure will prevent or significantly lessen the severity of smallpox symptoms in the vast majority of people. Vaccination four to seven days after exposure can offer some protection from disease or may modify the severity of disease. Other than vaccination, treatment of smallpox is primarily supportive, such as wound care and infection control, fluid therapy, and possible ventilator assistance. Flat and hemorrhagic types of smallpox are treated with the same therapies used to treat shock, such as fluid resuscitation. People with semi-confluent and confluent types of smallpox may have therapeutic issues similar to patients with extensive skin burns.No drug is currently approved for the treatment of smallpox. However, antiviral treatments have improved since the last large smallpox epidemics, and studies suggest that the antiviral drug cidofovir might be useful as a therapeutic agent. The drug must be administered intravenously, however, and may cause serious kidney toxicity.





_parasitized_by_Acrodactyla_quadrisculpta_larva_(RMNH.INS.593867)_-_BDJ.1.e992.jpg)

